|
|
|||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||
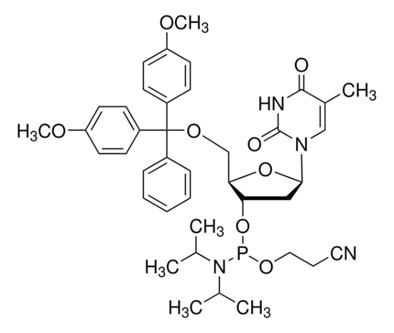 DMT-dT Phosphoramidite
DMT-dT Phosphoramidite
Catalog NO.: DNA-PM-006 | CAS NO.: 98796-51-1 | Brand: BIOCAXIS
Category
Carbohydrates, Nucleosides & Nucleotides, DNA Phosphoramidites
Synonyms:
5′-O-(4,4′-dimethoxytrityl)-2′-deoxythymidine-3′-O-[O-(2-cyanoethyl)-N,N′-diisopropylphosphoramidite], 5′-O-[bis(4-methoxyphenyl)phenylmethyl]-2′-deoxythymidine, 3′-[2-cyanoethyl N,N-bis(1-methylethyl)phosphoramidite], DMT-dT amidite, Thymidine, 5′-O-[bis(4-methoxyphenyl)phenylmethyl]-, 3′-[2-cyanoethyl N,N-bis(1-methylethyl)phosphoramidite]
Molecular Formula
C40H49N4O8P
Molecular Weight
744.81
General description
Proligo′s DNA phosphoramidites lead to high-yield and high-quality oligonucleotides due to their high coupling efficiency. The deprotection step of automated oligonucleotide synthesis is integral to synthesis time and final product quality. Exocyclic amine functions are protected by a benzoyl group (dA(bz) and dC(bz)) or isobutyryl group (dG(ib)). Recommended cleavage and deprotection conditions are 8 hours at 55 °C or 24 hours at room temperature using concentrated ammonia solution, for standard base-protected oligonucleotides.
Usage
Solid phase synthesis of DNA and RNA oligonucleotides in chemical or enzymatic processes.

