|
|
Phytol: Nature*s Versatile Biomolecule for
Health and Innovation
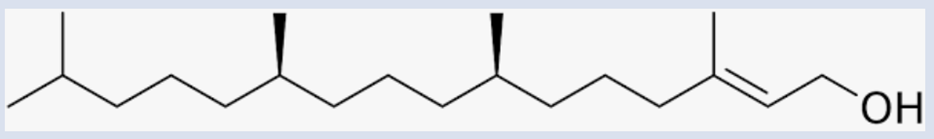
Phytol, a branched-chain diterpene
alcohol (C₂₀H₄₀O), is a ubiquitous
component of chlorophyll, the essential pigment driving photosynthesis in
plants, algae, and cyanobacteria (Fischer, 1928; Wilstätter, 1909). As a
hydrolysis product of chlorophyll, it is one of the most abundant acyclic
isoprenoids in the biosphere, offering a sustainable and naturally derived
resource for biomedical and industrial applications (Rontani & Volkman,
2003). At Biocaxis, we harness the potential of phytol through advanced
extraction and purification techniques, delivering high-purity phytol for
cutting-edge solutions in pharmaceuticals, nutraceuticals, and cosmetics.
Natural Origins and Biomedical Significance
Phytol*s unique structure underpins its diverse bioactivities. Studies
highlight its antioxidant, antimicrobial, and anti-inflammatory properties,
making it a candidate for therapeutic development (Islam et al., 2018; de
Moraes et al., 2014). For instance, phytol induces apoptosis and autophagy in
cancer cells, offering promise in oncology, while its anxiolytic effects
suggest applications in mental health (Kowalczyk et al., 2021). Additionally,
phytol derivatives, such as 污-butyrolactones, exhibit enhanced cytotoxic
activity against leukemia and lung carcinoma cells, underscoring its
versatility in drug design (Kowalczyk et al., 2021).
Industrial and Cosmetic Applications
Beyond therapeutics, phytol is a cornerstone in fragrances and cosmetics due
to its grassy aroma and stabilizing properties (Silva et al., 2024). It
serves as a precursor for synthetic vitamins E and K1, critical for skincare
and nutritional supplements (Al-Harrasi et al., 2019). Biocaxis prioritizes
eco-friendly extraction methods, sourcing phytol from renewable plant-based
materials to ensure sustainability and safety, avoiding risks associated with
animal-derived alternatives (Gutbrod et al., 2021).
Commitment to Innovation
Biocaxis leverages phytol*s molecular adaptability to develop tailored
formulations. Our phytol is rigorously tested for purity (>98%) and low
endotoxin levels, ideal for sensitive applications like gene therapy carriers
or functional food additives (Vetter & Schröder, 2010). Recent
advancements in enzymatic and chemical synthesis further enhance its bioavailability, enabling breakthroughs in metabolic
disorder treatments and antimicrobial agents (Silva et al., 2024).
Explore how Biocaxis*s phytol can elevate your product
pipeline, backed by cutting-edge research and a commitment to ecological
stewardship.
References
- Al-Harrasi,
A., et al. (2019). Acyclic
diterpenes. In Natural Product Biosynthesis (pp.
189每210). Elsevier. DOI: 10.1016/B978-0-12-816455-6.00009-3
- de
Moraes, J., et al. (2014). Phytol,
a diterpene alcohol from chlorophyll, as a drug against neglected
tropical disease schistosomiasis mansoni. PLOS Neglected Tropical
Diseases, 8(1), e2617. DOI: 10.1371/journal.pntd.0002617
- Fischer,
F. G. (1928). Structure of phytol.
Berichte der Deutschen Chemischen Gesellschaft, 61(1), 2400每2406.
- Gutbrod,
K., et al. (2021). Phytol derived
from chlorophyll hydrolysis in plants is a key intermediate in
stress-induced aldehyde accumulation. Journal of Biological
Chemistry, 296, 100530. DOI: 10.1016/j.jbc.2021.100530
- Islam,
M. T., et al. (2018). Phytol:
A review of biomedical activities. Food and Chemical Toxicology,
121, 82每94. DOI: 10.1016/j.fct.2018.08.032
- Kowalczyk,
M., et al. (2021). Synthesis
of novel phytol-derived 污-butyrolactones and evaluation of their
biological activity. Scientific Reports, 11, 4262. DOI: 10.1038/s41598-021-83736-6
- Rontani,
J. F., & Volkman, J. K. (2003). Phytol
degradation products as biogeochemical tracers in aquatic environments.
Organic Geochemistry, 34(1), 1每35. DOI: 10.1016/S0146-6380(02)00185-7
- Silva,
A., et al. (2024). New
phytol derivatives with increased cosmeceutical potential.
Molecules, 29(20), 4917. DOI: 10.3390/molecules29204917
- Vetter,
W., & Schröder, M. (2010). Phytanic
acid and pristanic acid in dairy products. Journal of Agricultural and
Food Chemistry, 58(1), 553每560. DOI: 10.1021/jf903065m
- Wilstätter,
R. (1909). Hydrolysis of chlorophyll.
Annalen der Chemie, 373(1), 177每204.
|
|